The Neuropathobiology of Multiple Sclerosis: A Pathway to Understanding Chronic Disability Progression
Multiple sclerosis (MS) is a complex immune-mediated and neurodegenerative disease that predominantly affects adults. Globally, it impacts nearly 2.8 million people, characterized by a complex interplay between inflammation, neurodegeneration, and neuronal dysregulation. Despite advancements in disease-modifying therapies that target inflammation, the path toward halting long-term disability progression in MS remains elusive. In this blog post, we will explore the recent breakthroughs in understanding MS's neuronal damage mechanisms and the implications for developing novel therapies.
The Shift from Relapses to Smoldering Disease Activity
Traditionally, MS has been classified into relapsing-remitting (RRMS) and progressive forms (SPMS and PPMS). However, recent studies indicate that neurodegeneration, particularly in progressive MS, occurs independently of overt relapses. This progression, termed "smoldering disease activity," includes low-grade inflammation and neuron-intrinsic deregulation, both of which perpetuate damage long after the initial inflammatory events.
For instance, studies have shown that disability accumulation occurs not only from relapse-associated worsening (RAW) but also from progression independent of relapse activity (PIRA), even in early-stage MS. This suggests that neurodegeneration starts early in the disease and proceeds alongside or independently of visible relapses, highlighting a gap in therapeutic targeting.
Neuroinflammation and Neuronal Damage
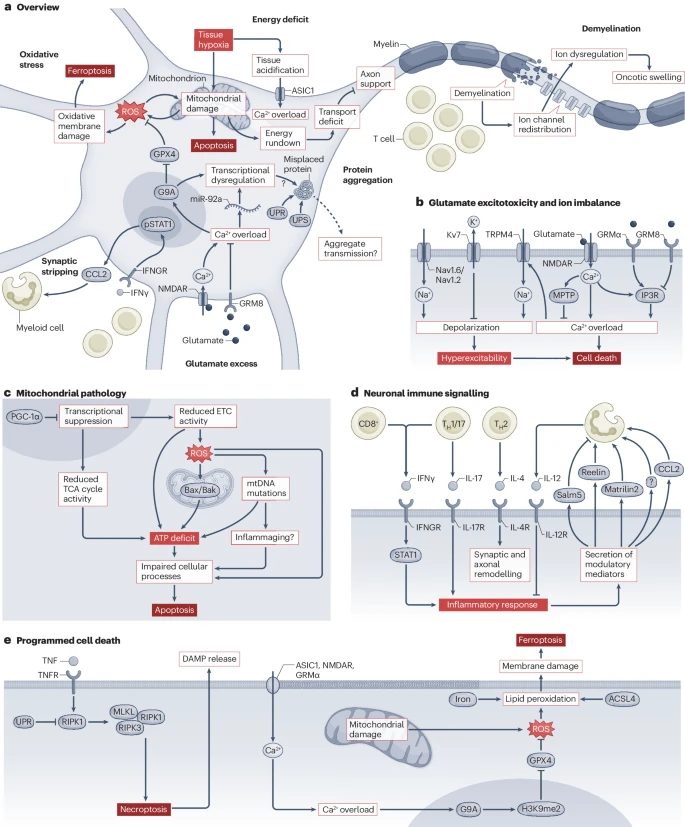
Central to MS pathology is the inflammatory milieu within the central nervous system (CNS), which leads to a cascade of neurotoxic events. The inflammatory environment disrupts neuronal homeostasis by inducing excitotoxicity, ion dysregulation, and oxidative stress, culminating in mitochondrial dysfunction and neuronal death. Excitotoxicity, for example, results from the excessive release of glutamate, which overstimulates N-methyl-D-aspartate (NMDA) receptors, causing an influx of calcium ions and subsequent neuronal damage. In this environment, mitochondria, essential for maintaining neuronal energy homeostasis, become overwhelmed, leading to cellular energy deficits and eventual cell death.
Mitochondrial Dysfunction: An Early Event in MS
Recent studies have pinpointed mitochondrial dysfunction as a key player in the early stages of MS-related neurodegeneration. Inflammation-induced energy shortages place neurons under significant stress, contributing to mitochondrial damage. Impairments in the respiratory chain and reductions in oxidative phosphorylation have been observed in both animal models and human MS studies. Notably, mitochondrial dysfunction often precedes observable neurodegeneration, making it a potential early marker for disease progression.
This mitochondrial distress results in the accumulation of reactive oxygen species (ROS) and decreased ATP production, which further exacerbates neurodegeneration. Research has shown that enhancing mitochondrial resilience, such as through genetic modifications that improve mitochondrial function, could alleviate some of the neuronal loss seen in MS.
Amplifiers of Neurodegeneration: Neuron-Intrinsic Pathways
While inflammation triggers the initial neurodegenerative cascade, the process is perpetuated by neuron-intrinsic pathways that feed into the damage. For example, neurons exposed to chronic inflammation activate internal stress pathways, such as the unfolded protein response (UPR), which can lead to protein aggregation and cell death. These pathways not only promote continued neuronal dysfunction but also signal nearby glial cells to amplify inflammation, creating a vicious cycle of damage.
This neuron-centric approach to MS highlights that neurodegeneration in MS is not solely a consequence of immune activity but also involves neuron-specific dysfunctions that sustain long-term disability.
Biomarkers of Neurodegeneration: NfL and GFAP
Emerging biomarkers offer new insights into the early detection and monitoring of neurodegeneration in MS. Neurofilament light chain (NfL), a marker of axonal damage, has been found to be elevated in both plasma and cerebrospinal fluid (CSF) during relapses and even before clinical symptoms emerge. Similarly, glial fibrillary acidic protein (GFAP) reflects astrogliosis, an inflammatory response associated with neurodegeneration. These biomarkers not only predict disease progression but may also serve as critical tools for evaluating the effectiveness of neuroprotective treatments in clinical trials.
Therapeutic Potential: Targeting Neuronal Pathways
Given the complexity of MS neurodegeneration, a promising avenue for treatment lies in targeting neuronal-specific pathways. Approaches aimed at modulating ion channels, reducing glutamate excitotoxicity, and enhancing mitochondrial function have shown neuroprotective potential in preclinical models. For instance, the inhibition of excitatory receptors or the enhancement of neuronal resistance to oxidative stress could alleviate some of the damage caused by chronic inflammation. Additionally, clinical trials targeting mitochondrial resilience and neuroprotective strategies are currently underway.
Conclusion: A Neuron-Centric Future for MS Therapies
Despite the availability of therapies that reduce inflammation and clinical relapses, the challenge of preventing chronic neurodegeneration in MS remains significant. The neuron-centric understanding of MS sheds light on the intrinsic factors that make neurons vulnerable to inflammation and offers hope for developing therapies that directly target the neuronal components of the disease. By focusing on the resilience of neurons and preventing the amplification of neurodegeneration, future treatments may be able to slow, or even halt, the progression of disability in MS patients.
Reference:
Woo, M.S., Engler, J.B. & Friese, M.A. The neuropathobiology of multiple sclerosis. Nat. Rev. Neurosci. 25, 493–513 (2024).
