The Immunology of Multiple Sclerosis: A Complex Interplay of Genetics, Environment, and Immune Responses
Multiple sclerosis (MS) is a complex immune-mediated neurodegeneration disease that primarily affects adults, with over 2.5 million people diagnosed worldwide. The unpredictability of its clinical course and the diversity of symptoms make diagnosis and treatment particularly challenging. Although genetic susceptibility plays a significant role, accounting for more than 30% of overall disease risk, environmental factors such as infections, nutrition, and vitamin D levels are critical in facilitating disease development in genetically predisposed individuals.
Genetic Susceptibility and Immune System Interactions
Multiple sclerosis (MS) is an autoimmune disorder characterized by inflammation, demyelination, and neurodegeneration. Affecting approximately 2.5 million people worldwide, MS is more prevalent in young adults and occurs more frequently in women than men. The geographic distribution of MS is notable, with higher incidence rates observed in temperate regions, such as North America and Europe, compared to tropical areas.
Genetics play a significant role in determining an individual's susceptibility to MS, though environmental factors such as vitamin D levels, viral infections (e.g., Epstein-Barr virus), and smoking also contribute. Recent advances in genetic research have shed light on several key genes and loci associated with MS risk, including the human leukocyte antigen (HLA) region on chromosome 6. The HLA-DRB1*15:01 allele, in particular, has been identified as a major genetic risk factor, with carriers being three to four times more likely to develop MS.
Genetic Susceptibility and Immune System Interactions
The strongest genetic risk factor for MS is found in the human leukocyte antigen (HLA) complex, with several HLA class II alleles (e.g., HLA-DRB1*15:01) strongly associated with increased disease risk. These HLA alleles facilitate the presentation of myelin antigens to autoreactive T cells, thereby initiating an immune attack on the central nervous system (CNS). While CD4+ T cells are central to this process, targeting these cells for therapeutic intervention has yielded limited clinical benefit, suggesting that the pathogenesis of MS involves more than just autoreactive T cells.
In addition to HLA-related genetic factors, genome-wide association studies (GWAS) have identified over 200 non-HLA loci that contribute to MS risk. Most of these loci are involved in immune regulation, with many residing in non-coding regions of the genome, influencing the expression of genes in immune cells. Epigenetic modifications further complicate this picture, with environmental factors such as infections and nutrition modulating the activity of immune-related genes.
The Role of Environmental Triggers
One of the most compelling environmental risk factors for MS is infection with the Epstein–Barr virus (EBV). Studies have shown that individuals infected with EBV, particularly those who develop infectious mononucleosis, are at a significantly higher risk of developing MS later in life. In fact, EBV infection increases the risk of MS by 32-fold, highlighting the critical interplay between viral infections and genetic predisposition. Other herpesviruses, such as human herpesvirus 6 (HHV-6), have also been implicated in MS, further underscoring the importance of viral infections as environmental triggers.
Immune System Dynamics in MS
The immune system plays a dual role in MS, contributing to both disease onset and progression. Early in the disease, the adaptive immune system, particularly CD4+ and CD8+ T cells, is thought to drive autoimmune attacks against myelin, the protective sheath around neurons. CD4+ T helper cells (TH1 and TH17) are frequently found in MS lesions, and their polarization towards pro-inflammatory states is a key factor in disease pathogenesis.
However, the involvement of other immune cells, such as natural killer (NK) cells and innate lymphoid cells (ILCs), suggests that the immune response in MS is more complex than initially believed. NK cells, for example, may play both protective and pathogenic roles, depending on their activation state and environmental context. Similarly, B cells have emerged as important players in MS, with therapies targeting B cells, such as anti-CD20 monoclonal antibodies, proving highly effective in reducing MS relapses.
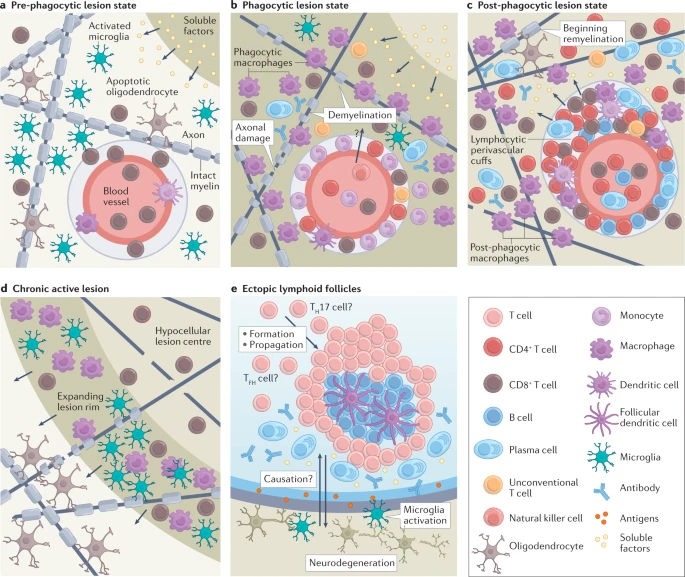
CNS Immunopathology and Disease Progression
MS is characterized by the formation of lesions in the CNS, particularly in the white matter, where myelin is attacked by the immune system. These lesions are associated with both physical disability and cognitive decline. The immune cells that infiltrate the CNS during MS include not only T and B cells but also monocytes, macrophages, and microglia, the resident immune cells of the CNS. Microglia, in particular, play a crucial role in maintaining tissue homeostasis and regulating immune responses within the CNS. However, in MS, they can become overactivated, contributing to the progression of neurodegeneration.
Interestingly, the discovery of an intracranial lymphatic network has challenged the notion that the CNS is an immune-privileged organ. This network allows for the drainage of cerebrospinal fluid and immune surveillance of CNS antigens, which may play a role in the immune dysregulation seen in MS.
Therapeutic Implications
The treatment of MS has evolved significantly over the past few decades, with current therapies focusing on modulating the immune system to reduce inflammation and prevent further damage to the CNS. However, most of these treatments are not disease-specific and act as broad immunosuppressants, leading to a range of potential side effects. Emerging therapies aim to target specific immune pathways, such as blocking the pro-inflammatory cytokine GM-CSF or enhancing the regulatory functions of T cells and B cells.
Understanding the plasticity of immune cells in MS, and how they can shift between protective and pathogenic states, is crucial for developing more effective treatments. For example, the ability to induce regulatory T cells (Treg cells) through short-chain fatty acid supplementation or vitamin D therapy offers a promising avenue for reducing disease activity without broad immunosuppression.
Conclusion
Multiple sclerosis is a multifaceted disease driven by a complex interplay of genetic susceptibility, environmental triggers, and immune system dysregulation. While significant progress has been made in understanding the immunological mechanisms underlying MS, much remains to be discovered, particularly regarding the roles of non-traditional immune cells and the impact of environmental factors. As research continues to uncover new therapeutic targets, the hope is that more precise and effective treatments will become available, improving the quality of life for individuals living with MS.
Reference:
Attfield, K.E., Jensen, L.T., Kaufmann, M. et al. The immunology of multiple sclerosis. Nat Rev Immunol 22, 734–750 (2022).
