A Detailed Look into Human Disease Genetics: A Scientific Overview
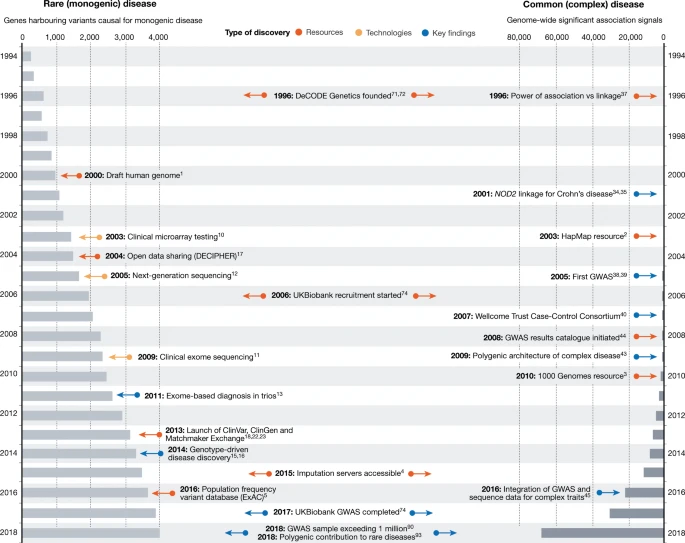
Over the past 25 years, human genetics has been revolutionized by technological advancements, fostering breakthroughs in understanding the genetic underpinnings of disease susceptibility. The central theme of the paper, A Brief History of Human Disease Genetics published in Nature, emphasizes how disease genetics has evolved, revealing insights into both rare monogenic disorders and common complex diseases. This journey showcases how the confluence of genomic tools and large-scale biobanks is reshaping medical innovation through the lens of precision medicine.
Historical Milestones in Genetic Discoveries
The quest to map disease-related genes began with rare, monogenic diseases—genetic conditions caused by mutations in a single gene. In the 1980s and 1990s, linkage analysis in large families with specific genetic disorders, such as Huntington’s disease and cystic fibrosis, drove many of these discoveries. However, these methods were laborious, and the completion of the Human Genome Project in 2001 acted as a catalyst, drastically reducing barriers to gene identification.
In parallel, genome-wide association studies (GWAS) gained traction as a method for identifying common genetic variants that contribute to complex diseases. Unlike rare diseases, these common diseases—like diabetes, heart disease, and asthma—result from the interaction of multiple genetic variants, each with a modest effect. GWAS has enabled researchers to pinpoint thousands of loci associated with these traits, transforming our understanding of polygenic inheritance.
Rare Variants and Disease Causality
The identification of rare disease-causing variants has surged with the advent of high-throughput sequencing technologies, including exome and genome-wide sequencing. The ability to sequence an individual's entire coding region (exome) or genome has unveiled previously undetectable structural variants, from small indels to large chromosomal rearrangements. This has accelerated genetic discovery, especially in populations with diverse genetic backgrounds.
Beyond identifying causal variants, researchers now strive to understand the variable penetrance of these alleles—how and why some individuals with a disease-causing mutation exhibit symptoms while others remain unaffected. This is particularly relevant in the context of monogenic disorders, where the clinical manifestation can vary widely. It is likely that genetic modifiers and environmental factors play a role in these differences, highlighting the complex interplay between genetics and environment.
The Evolution of Genome-Wide Association Studies (GWAS)
GWAS has been pivotal in unraveling the genetic architecture of common diseases. However, early efforts were hampered by small sample sizes and poor replication. With the advent of larger biobanks, such as the UK Biobank, which now includes over 500,000 individuals, the power of GWAS has multiplied, allowing for more accurate identification of genetic variants associated with disease.
Although many of the common variants discovered through GWAS are non-coding, and their functions are unclear, the integration of functional genomic datasets—such as the ENCODE project—has provided crucial insights into how these variants may affect gene regulation. By mapping regulatory elements in various tissues and cell types, researchers have begun to understand how genetic variants exert their effects at the molecular level.
The Role of Biobanks and Population Studies
Large-scale population biobanks have been essential in expanding our understanding of the genetic basis of disease. These biobanks, including the UK Biobank and others in North America, Asia, and Europe, provide a wealth of data, combining genetic information with a wide range of phenotypic data, including clinical records, lifestyle factors, and biochemical measurements.
Importantly, these biobanks allow for longitudinal studies, where participants are followed over time to track the development of diseases and other health outcomes. This approach provides invaluable insights into how genetic risk factors interact with environmental and lifestyle factors to influence disease risk over a lifetime.
Polygenic Risk Scores and Precision Medicine
One of the most promising applications of genetic research in clinical practice is the development of polygenic risk scores (PRS). These scores aggregate the effects of many common variants across the genome to estimate an individual's genetic risk for a given disease. PRS have already been developed for a range of diseases, including cardiovascular disease, diabetes, and cancer.
For example, a PRS for coronary artery disease can identify individuals with a genetic risk equivalent to those with rare monogenic forms of hypercholesterolemia. As the accuracy of these scores improves with larger datasets and better algorithms, PRS could become a cornerstone of personalized medicine, enabling tailored screening, prevention, and treatment strategies based on an individual's genetic risk.
Looking Forward: Challenges and Opportunities
While tremendous progress has been made in human disease genetics, several challenges remain. A key challenge is the underrepresentation of non-European populations in genetic studies, which limits the generalizability of findings. Expanding genomic research to include more diverse populations is critical to ensuring that the benefits of genetic discoveries are shared equitably.
Moreover, functional studies are needed to validate the effects of disease-associated variants and to understand the molecular mechanisms that underlie genetic risk. New technologies, such as CRISPR-based genome editing and single-cell transcriptomics, offer exciting opportunities to explore the functional consequences of genetic variation in unprecedented detail.
Conclusion: A New Era of Genomic Medicine
The past quarter-century has seen remarkable advances in human disease genetics, from the discovery of rare disease-causing variants to the identification of thousands of common variants associated with complex diseases. These discoveries have laid the foundation for a new era of precision medicine, where healthcare can be tailored to an individual's genetic makeup.
As genomic technologies continue to evolve and biobanks grow in scale, we are on the cusp of even greater breakthroughs. The challenge now is to translate these genetic insights into clinical practice, ensuring that the benefits of genomic medicine are realized for all individuals, regardless of their genetic background or geographic location.
Reference:
Claussnitzer, M., Cho, J.H., Collins, R. et al. A brief history of human disease genetics. Nature 577, 179–189 (2020).
